Rabbit Skeleton
EXAMINATION OF FETAL SPECIMENS - OVERVIEW
This training web has been designed as a programme of training and assessment to support the Society of Biology's International Register of Fetal Morphologists. It provides learning material that will complement practical training received 'in house.
It is preferable that the candidate attends the 'Introduction to fetal morphology' presentation prior to, or shortly after, commencement of practical training.
To conduct fetal examinations adequately, it is important to have a clear idea of the normal anatomy and of how to describe variations from the norm. Apart from basic knowledge of the relevant fetal anatomy, some broader background or training in embryological development will also be very valuable.
The training web presents important topics as 'learning objectives' and uses images and diagrams to demonstrate current popular practices and conventions. The candidate should, however, always refer to their own laboratories Standard Operating Procedures (SOPs) and user guides and use this training web alongside their own material.
The candidates understanding of the learning objectives can be assessed using the 'learning outcome' exercises provided.
The material contained in this website provides training and guidance relevant to RABBIT SKELETAL EXAMINATION.
RABBIT SKELETAL EXAMINATION - INTRODUCTION
Why are fetuses examined?
The aim of regulatory reproductive toxicity studies is to reveal any effect of an active substance on mammalian reproduction. In one of the studies designed to achieve this aim pregnant animals are treated during the period of embryonic and/or fetal development.
Current International Conference on Harmonisation (ICH), Organisation for Economic Co-operation and Development (OECD) and the US Environmental Protection Agency (EPA) guidelines require that these embryofetal development studies are performed in 2 laboratory animal species (one rodent and one non-rodent), and that fetuses from the treated dams are examined for developmental and structural abnormalities, by soft tissue and skeletal examination techniques.
The rabbit is commonly chosen as the non-rodent species and the current guidelines, referred to above, specify that all of the specimens should be examined skeletally.
Rabbit specimens are commonly stained with either alizarin red S alone or in combination with alcian blue. Both methods produce robust, clear, articulated specimens which can be easily manipulated and examined many times, if necessary.
Training to examine skeletons
Ideally the trainee should be provided with a training plan and the date of any training received should be recorded. Regular review of the trainee's progress is essential.
SOPs and user guides should be made available as training materials. These should be referred to, along with any other relevant materials, such as this training web, during training. Adherence to Good Laboratory Practices should be shown at all times.
Initial teaching sessions are ideally organised on a one-to-one basis, interspersed with time allowed for the trainee to practice, consolidating what has been learned. Wherever possible, the use of materials from studies that have already been evaluated will avoid time pressures at this stage.
Once this initial phase has been completed, training can then be continued 'on-study' with close supervision by experienced examiners. Initially, training should include 100% review of the examinations performed by the trainee, gradually reducing as competency is acquired. The time taken to achieve competency will naturally depend on work throughput, as much as on the aptitude of the trainee, and it might be necessary to revert to more exhaustive checking if checking/review reveals an increasing level of inaccuracy or inconsistency. It is expected that the trainee would have 18 months experience in the technique, and have evaluated a minimum of 500 litters before they are be considered fully competent.
It is recommended that all structures listed on the The International Register of Fetal Morphologists (IRFM) Expected Minimum Structure List for Fetal Morphology Examinations document be examined and that the Skeletal examination - key structures and notes document be used as a reference.
Learning objectives:
- Familiarise yourself with your training objectives
- Review 'in house' training material
- Review UK IRDG recommended minimum requirements for fetal examinations document
Requirements for examination
The skeletal elements of relatively large specimens such as rabbits can be visualised using the naked eye, however, the use of a low power magnifier and immersion in fluid are recommended. A good light source is also essential - the specimen may be illuminated from above, from below or a combination of the two. The setup pictured below demonstrates the specimen being illuminated from below.

The specimen can be moved and manipulated using fingers, forceps or a combination of the two, the examiner should find what is comfortable for them whilst allowing free movement.
Since there is no absolute and wholly objective standard against which to evaluate specimens, every effort should be made to assess the anatomy in exactly the same way for every specimen, applying identical criteria to distinguish 'normal' from 'abnormal' for the gestational age being examined. It is recommended that measures to ensure a consistent approach be built in to any laboratory's work practice. Working methodically should increase the consistency of examination and limit the likelihood of omissions. Whether all examiners in a laboratory should be required to follow the same routine or be allowed to develop their own is a matter for individual preference, but some degree of consistency is desirable.
Remember:
Magnification
- Magnifier x2 minimum recommended
Light source
- Specimen lit from above and/or below
Examination dishes
- Clear deep containers, able to contain 2 specimens
Dissection equipment
- Forceps (curved or straight)
Laboratory work station
- In quiet well ventilated room, specific for purpose
- Adequate space for:
- manipulation of specimen under examination
- whole litter being examined
- container for medium in which specimens are examined e.g. glycerol
- paper towels
- data collection system
- paper records or
- monitor and keyboard
- Suitable chair
- Comfortable, safe
- 3 way adjustable
- Lighting
Access to reference material
- UK IRDG terminology manual
- The International Register of Fetal Morphologists (IRFM) Expected Minimum Structure List for Fetal Morphology Examinations
- Terminology in Developmental Toxicology glossary
- Company training guides, including recognition levels employed
- Company SOPS
- Examples of normal specimens
PREPARATION OF SPECIMENS
Eviscerated specimens may be stained using either alizarin red S or a combination of alizarin red S and alcian blue.
Alizarin red S selectively binds to calcium, which is present in ossified bone, and stains it red. alcian blue selectively stains mucopolysaccharides present in cartilaginous tissue blue.
Typically specimens which are to be stained using alizarin red S only are immersed in potassium hydroxide (which macerates connective tissue), stained and then cleared using glycerol to leave an articulated skeleton with stained ossified bone. Double staining methods are more complicated and require more specimen preparation.
Learning objective: Take a look at these staining methods and compare them with the method(s) used in your own laboratory.
As the extent and density of staining are used as an indicator of the stage of ossification of a bone, it is very important that specimens are evenly stained both within and across studies. This should ensure that, within any one laboratory, consistent observations can be made.
Problems may result if the specimen has not been completely immersed in processing fluids or stain, resulting in ossified areas being unstained, or if the specimens are clumped together, causing non-uniform penetration of the stain.
The method used in your laboratory may render fat translucent, if it doesn't the fatty tissue, especially that present on the dorsal surface will have to be removed manually. This increases the chances of mechanical damage to the specimen but is essential if the skeleton is to be examined satisfactorily.
Formation of bone
Before looking at the skeleton itself it is worth looking at how bone is formed, an understanding of this process will enable a better understanding of ossification patterns seen at examination. The examples shown below relate to bone formation in the human skeleton but are equally applicable to other mammals.
Bone is formed either by direct ossification of embryonic connective tissue (intramembranous ossification) or by replacement of hyaline cartilage (intracartilaginous or endochondral ossification). Intramembranous ossification takes place in the so-called membrane bones of the skull, while endochondral ossification is characteristic of the bones of the trunk and extremities.
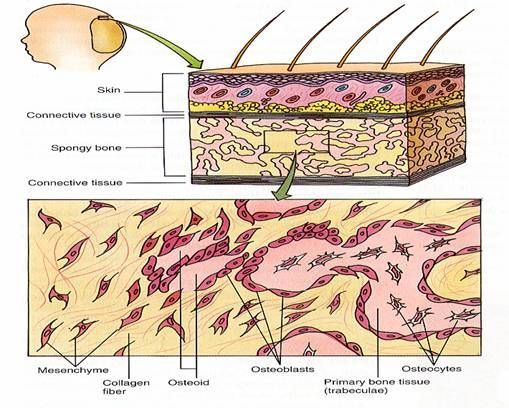
There are several events, which take place in intramembranous bone formation. The events are show in Figure 3 above and listed as follows:
a. Increased vascularity of tissue.
b. Active proliferation of mesenchymal cells. The mesenchymal cells give rise to osteogenic cells, which develop into osteoblasts.
c. Osteoblasts begin to lay down osteoid. Osteoid is the organic part of bone without the inorganic constituent.
d. Osteoblasts either retreat or become entrapped as osteocytes in the osteoid.
e. The osteoid calcifies to form spicules of spongy bone. The spicules unite to form trabeculae. The inorganic salts carried in by the blood vessels supposedly bring about calcification. The salts are deposited in an orderly fashion as fine crystals (hydroxyapatite crystals) intimately associated with the collagenous fibers. These crystals are only visible with the electron microscope.
f. Bone remodeling occurs. Periosteum and compact bone are formed.
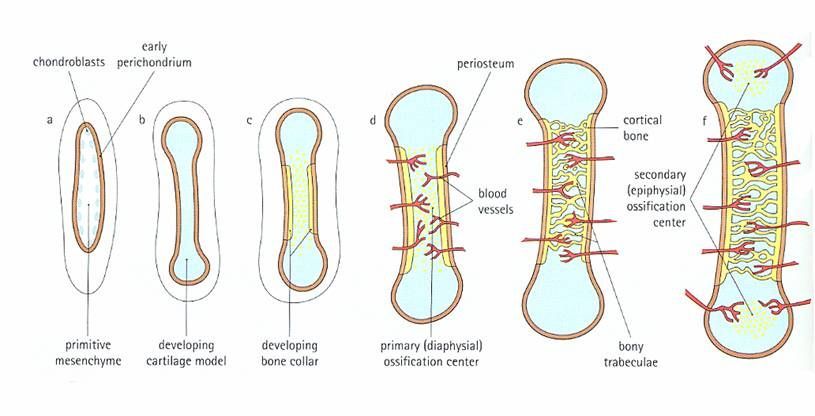
Intracartilaginous (endochondral) bone formation.
This type of ossification involves the replacement of a cartilaginous model by bone and is best observed in long bones, such as the humerus or femur. Events of endochondral ossification can be seen in the figure below below and include the following:
a. Primary ossification center. The first change indicative of beginning ossification takes place about the center of the future bone shaft. Here the cartilage cells hypertrophy and the cartilage matrix becomes calcified. Subsequently, part of the calcified matrix disintegrates, opening cavities that communicate with the connective tissue and vessels at the surface.
b. Bone collar. The bone collar forms concurrently with the primary ossification center. Cells of the perichondrium begin to form bone. The bone collar holds together the shaft, which has been weakened by the disintegration of the cartilage. The connective tissue about the bone collar, previously a perichondrium, is now called periosteum.
c. Periosteal buds. These are connective tissue buds or "sprouts" containing mesenchymal cells (which give rise to osteogenic cells) and blood vessels, which grow from the periosteum to reach the primary ossification center. Osteoblasts attach to spicules of calcified cartilage in the primary ossification center and begin to produce osteoid. Thus, bone is formed and the process continues toward both epiphyses while this is occurring, the cartilage outside the primary ossification center increases in size by interstitial and appositional growth.
d. Secondary ossification centers. About the time of birth, a secondary ossification center appears in each end (epiphysis) of long bones. Periosteal buds carry mesenchyme and blood vessels in and the process is similar to that occurring in a primary ossification center. The cartilage between the primary and secondary ossification canters is called the epiphyseal plate, and it continues to form new cartilage, which is replaced by bone, a process that results in an increase in length of the bone. Growth continues until the individual either reaches maturity or the cartilage in the plate is replaced by bone. The point of union of the primary and secondary ossification centers is called the epiphyseal line.
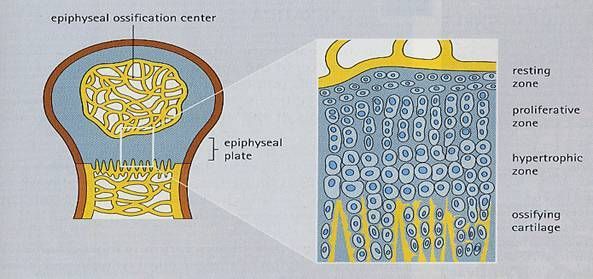
There are five zones of cartilage associated with endochondral ossification.
1. Zone of reserve cartilage. This is typical hyaline cartilage and is a large zone in this preparation.
2. Zone of cell proliferation (ZP). The cartilage cells are small and tend to be arranged in columns, which run parallel to the long axis of the cartilage. This arrangement is indicative of their intense mitotic activity.
3. Zone of cell and lacunar maturation and hypertrophy enlargement (ZH). Chondrocytes and lacunae are larger than in the previous zone. The chondrocytes increase in size and resorb some their lacunar walls, enlarging them to such an extent that some of the lacunae become confluent.
4. Zone of calcification (ZC). This is a small zone having a slightly darker appearance than the preceding zone due to the basophilic staining of the calcified cartilage. The chondrocytes die in this zone.
5. Zone of cartilage removal and bone deposition. Osseous elements are present among the pieces of calcified cartilage.
Learning objective: The first bones in the fetal skeleton to show calcification are the clavicles. To understand more about the order of ossification in the fetal rabbit skeleton review the paper by Fritz (1975).
ASSESSMENT OF NORMAL
It is considered important to remember that if the examination data are collected inaccurately, inconsistently, or with insufficient detail, meaningful interpretation of these data is impossible. The examination of fetal specimens is therefore a crucial step in the evaluation of reproductive toxicity studies. The importance of this stage in data evaluation cannot be overstated.
Recognition of the 'normal' appearance
Laboratory recognition levels are the cut-off points between what is considered to be 'normal' and what must be recorded as 'abnormal' within a particular laboratory over a particular time period. Also, where observations are qualified by estimates of extent ('severity'), recognition levels provide the cut-off points between severities (advice on the recording of severity is given below). Translated into practical terms, they are criteria enabling the examiner to make consistent decisions at the borderlines between different categories.
Between laboratories there can be significant differences in the recognition levels set for some observations, in particular those relating to incomplete ossification. It would be difficult to attempt 'standardization' across laboratories in this area, because this would require setting recognition levels at an unacceptably coarse level in order to allow for the many factors that lead to inconsistency (including strain of animal, time of necropsy, laboratory environment and procedures). The priority should be ensuring consistency within each laboratory, so that valid comparisons can be made between treated and control animals, both within and between studies conducted in that laboratory.
Deciding where to draw the line between what is considered unremarkable and what must be recorded as an observation is one of the most difficult problems in fetal examination. In all biological systems there is natural variation and very few individuals in a population, or in a population sample, will have a body conformation that complies precisely with any idealized textbook description (the anatomical norm or anatomical standard). Most individuals will have an appearance that differs slightly from the anatomical norm, but which does not do so sufficiently, nor so infrequently, as to be regarded as 'abnormal'. This range of conformity can be considered to constitute the population norm.
Each laboratory uses recognition levels in the recording of fetal abnormalities, which define the boundaries between what is considered to be normal and what is abnormal within that laboratory, and also in many cases the boundaries of the qualifications used to describe the extent of an abnormality.
Other considerations - Assessing mechanical damage and processing artefacts
It is possible for mechanical damage to occur at necropsy, or for other artefacts to be introduced during processing of the fetal skeleton. It is also possible for incidental mechanical damage to occur in utero, although it must be borne in mind that in utero mechanical effects can also be induced by treatment (e.g. distortions produced by compression of the fetus by the uterine smooth muscle).
It is strongly recommended that any damage known to have occurred during the stages before fetal examination is recorded and that appropriate notes are made available to the skeletal examiner. Where conditions observed at skeletal evaluation are suspected but not confirmed to be artefactual, evaluation requires a common-sense approach. Examples of questions to be considered are:
- Are the margins of the structure smooth or jagged, as if shearing has taken place?
- Can the pieces be 'jig-sawed' back together?
- Has excessive maceration taken place, resulting in break-down of the soft tissues holding the skeleton together, particularly the extremities, such that bones have become detached? (Have a look in the bottom of the jar!)
- Has the specimen not been completely immersed in processing fluids or stain, resulting in ossified areas being unstained, or have the specimens clumped together, causing non-uniform penetration of the stain?
- Has the specimen been fixed in a posture that has caused the rib cage to appear asymmetrical, the pelvis to appear out of alignment, or the limbs to appear distorted?
Click here to view the normal rabbit skeletal specimen.
ASSESSMENT OF DIFFERENCES FROM NORMAL
Changes from normal should be recorded according to your own laboratory's standards, with references to internal recognition levels, using the appropriate SOPs, training guides, user manual and any other relevant training information.
It is recommended that some means of categorizing the degree of change or extent of an observation be devised to aid in interpretation. Commonly used systems use qualifiers such as minimal (or slight), moderate and marked (or severe). Defining these terms without specific examples is difficult, but the following general principles might be suitable:
- Minimal: Apparent at close scrutiny
- Moderate: Apparent without close scrutiny
- Marked: Strikingly obvious
Not all observations need to be qualified by severity, but where a single term might be descriptive of a wide range of severity (from a condition which is only slightly outside the normal range to one which is potentially much more harmful), a qualifier indicating severity is strongly recommended. Examples of such terms are: misshapen, displaced, enlarged, reduced in size, shortened and widened.
Where severities are used, they should be noted at the examination stage, but where appropriate they can be merged or rationalized at a later data evaluation stage. The data must be assessed carefully to ensure that such a rationalization will not obscure or exaggerate any findings.
Perhaps the most common observation recorded at fetal examination is incompletely ossified. This observation indicates that the extent or density, or both, of stain within the affected structure is less than expected. To make such an observation, it is imperative that the examiner is aware of what to expect under his/her laboratory conditions.
The appearance of structures that are incompletely ossified can vary considerably. Some examples follow:
- Absence of stain at the margins of a sheet of bone or at one or both of the extremities of a linear bone (i.e. reduction in the extent of the stained portion)
- Absence of stain in localized area(s) within a sheet of bone, or between the two extremities of linear bone
- Localized patches of faint and darker (normal) stain
- Faint stain throughout
To describe such differences, some examiners might use qualifying terms in addition to the generic term incompletely ossified. Actual examples of the use of such qualifiers are illustrated below:
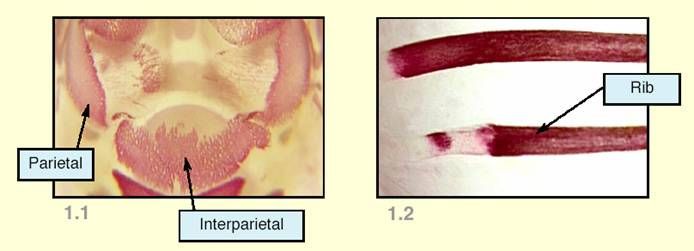
Figure 1.1 shows incomplete ossification where the extent of the stained area is reduced, particularly because of absence of stain at the medial margins of the parietals and the lateral margins of the interparietal. The depth of colour of the stain is also uneven. To describe this condition, qualifying terms such as irregular ossification, patchy ossification, or uneven ossification might be used.
Figure 1.2 shows incomplete ossification where there is absence of stain in a localized area between the extremities of a bone. To describe this condition, the qualifying term interrupted ossification might be used.
Examples of other qualifying terms that could be used in conjunction with incompletely ossified are:
- Diffuse ossification - denoting faint stain throughout a structure (e.g. cranial bones)
- Discontinued ossification - denoting premature termination of the stained area of a structure (e.g. a rib)
If deficiencies in the extent and density of staining are not the result of artefact, they might be due either to a delay in the ossification process, or to a flaw in the underlying skeletal structure. Because fetal examination represents only a snap-shot in time, it will be uncertain whether ossification in such cases will remain insufficient or will ultimately become complete.
Variations in ossification with two primary centres
There are some bones, or constituents of bones, that arise from two primary ossification centres which subsequently fuse. This ossification process is preceded by a similar process of fusion between two chondrification centres that form the underlying cartilaginous precursor. Examples of bones which form in this way are the supra-occipital, the vertebral centra and the sternebrae. In each of these structures, the two primary ossification centres are located on either side of the midline of the mature structure and, where this is the case, the following observations are quite common in rabbit fetuses at Day 29 of pregnancy:
- Unfused ossification centres - two separate stained areas, usually arranged symmetrically on either side of the centre of the expected area of ossification. This condition is often incorrectly described in regulatory study reports: Terms such as bipartite, cleft, split, or schisis are used, but are inappropriate, because they imply that the bone precursor is split into two parts, when in fact the appearance of two centres is merely caused by the onset of ossification in two discrete areas of a normal precursor.
- Incompletely fused ossification centres - two centres, as above, but partially fused. This observation might have several gradations, from connection of the two centres by a very thin bridge of ossification to a wide area of fusion such that the appearance of the structure is close to normal.
Two other observations that can be used to describe ossification irregularities in bones which develop from two primary centres are:
- Asymmetrically ossified - where development of the two parts has not proceeded at the same rate, such that disparity in the size of the two components of the stained area has led to asymmetry. This can apply to structures showing unfused ossification centres, to any gradation of incompletely fused ossification centres and also to normally ossified structures where full fusion has occurred.
- Unilateral ossification - where stain uptake has occurred in one primary ossification centre but not the other. In this case, it is important to establish whether the cartilaginous precursor is present in the unossified part and hence, whether ossification is ultimately likely to occur (or not) in both sides of the structure.
Where a bone or the constituents of a bone arise from two ossification centres, it is important to try to distinguish between a slight disturbance of the normal developmental process and a more serious developmental arrest. An example of the former would be where the underlying cartilage precursor has formed normally, but the two ossification centres have not fused or are incompletely fused. An example of the latter would be failure of both the cartilaginous precursors and the ossification centres to fuse completely, in which case it is more likely that there will be a permanent midline deficiency in the structure.
In specimens which have been stained only with alizarin red S, the appearance of these two conditions might be very similar. In these circumstances, it is important to draw upon other clues to try to distinguish between the two cases. Useful avenues of investigation are the positioning or shape of the two stained areas and/or the surrounding structures and it is important that any changes in these factors are noted. The spacing of the ossification centres is one possible clue; the wider they are apart, the more likely it is that the condition is consequent to disturbance of development of the precursor.
The shape and position of adjacent intervertebral discs are also important. Even without staining of the fibrocartilage, it is sometimes possible to see that these structures are misshapen and/or positioned such that they protrude into the area between the two ossification centres of the centrum. Where this is the case, the bone precursor could be abnormal and therefore a condition more serious than unfused ossification centres or incompletely fused ossification centres is suggested.
In cases such as these it is pointed out that, whilst general adherence to examination procedures and conventions of description is important for maintaining consistency, the application of 'common sense' is also necessary.
Examples of conditions in which contextual clues can help to distinguish between deviations in cartilage formation and the later process of ossification are given below.
Conditions requiring clarification:
- Is the structure incompletely ossified or is it interrupted, shortened, misshapen or reduced in size? (i.e. Is there a normal precursor which is incompletely ossified or is the precursor interrupted, shortened, misshapen or reduced in size?)
- Is the structure unossified or is it absent? (i.e. Is there a normal precursor with absent ossification or is the precursor also absent?)
- Is there an absent structure or are two structures fused?
Means of investigation:
- Is there sufficient room for a normally shaped bone to ossify?
- Can the precursor be seen or felt, or is there a gap or hole where all or part of the normally shaped (or sized) ossification centre would lie?
- Are the surrounding structures in the expected relative positions, or are they closer together or farther apart than expected, and is there a loss of symmetry?
- Are any of the surrounding structures misshapen, enlarged and/or tending to extend into the space normally occupied by the absent element(s) or part(s) of element(s)?
Counting the numbers of vertebrae within the different regions of the vertebral column is very important. Changes in vertebral configuration are indicative of disturbance of the axial gradient which lays down the template for formation of the spinal column. To a greater or lesser extent, variations in the number of vertebral elements can be found in all species. However, changes in the expected proportion of fetuses with such variations can provide a sensitive indicator of an effect of treatment.
In order to perform definitive counts, it is clearly necessary to be able to identify positively the borders of the five regions (cervical, thoracic, lumbar, sacral, caudal) of the vertebral column. In the adult animal this is relatively easy, because the general shape and characteristic processes of the vertebrae are different in the different regions, but in the rabbit fetus at Day 29 of pregnancy it is a little more difficult, because some of the distinguishing features are not ossified at this stage.
Some clues as to vertebral identification can be gleaned from the ossified elements and from the surrounding structures, the clearest examples being the cervicothoracic and thoracolumbar borders, which are generally distinguishable by the presence or absence of full ribs. However, other borders are less clear.
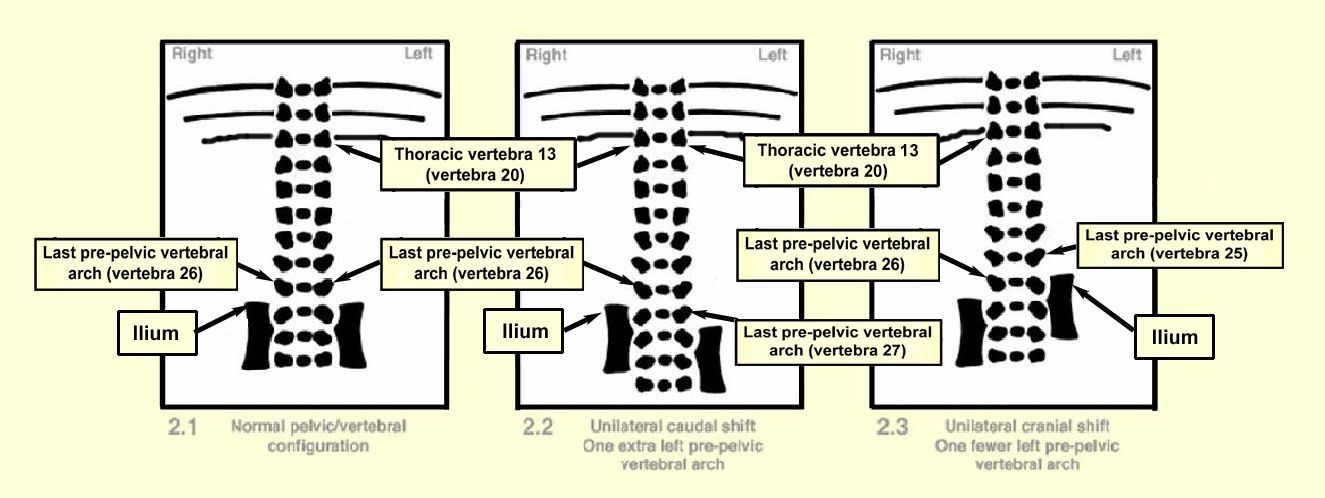
The usual way of identifying the lumbosacral border is to look for the point of articulation of the pelvic girdle which can be seen in more mature animals, such as the rabbit fetus, and counting the number of pre-pelvic vertebral arches. A series of diagrams is included below to show some features of the vertebral-to-pelvic relationship.
In the alizarin red S stained rabbit fetus at Day 29 of pregnancy, there are structures (articulation points) to confirm the position of articulation of the pelvic girdle. The lumbosacral border can usually be identified by means of two reference points. The first is a change in the alignment of the vertebral arches. The lateral margins of the last lumbar vertebral arches appear to point cranially, in contrast to the lateral margins of the first sacral vertebral arches, which point caudally. The second is the alignment of the iliac crests of the pelvic girdle, relative to the vertebral column. The iliac crests can be expected to lie approximately in line with the cranial margins of the first sacral vertebra (see Diagram 2.1 below).
These reference points can be used to identify variations in articulation of the pelvic girdle ('pelvic shift'). By observing the alignment of the vertebral arches and the iliac crests and then counting the total number of pre-pelvic vertebral arches, unilateral or bilateral shifts can be identified and described.
'Normal' rabbit fetuses have 26 pre-pelvic vertebrae (7 cervical, 12 thoracic, 7 lumbar), as shown in Diagram 2.1 below. Differences in this count are most commonly the result of minor changes in alignment of the pelvic girdle, sometimes associated with the presence of supernumerary ribs. For ease of recording unilateral as well as bilateral changes in pelvic alignment, counting the number of pre-pelvic vertebral arches on each side of the body appears to be the best practice. Conditions in which there are more than 26 pre-pelvic vertebral arches (as shown in Diagrams 2.2 and 2.4) are described as 'caudal' pelvic shift. Conditions in which there are fewer than 26 pre-pelvic vertebrae (as shown in Diagrams 2.3 and 2.5) are described as 'cranial' pelvic shift. Unilateral pelvic shift occurs when the position of pelvic articulation is changed on only one side of the body. Bilateral pelvic shift occurs when the position of pelvic articulation is changed on both sides of the body.
Diagrams 2.1 to 2.5 illustrate the ventral aspect of the thoracolumbar region and assume seven cervical and twelve thoracic vertebrae.
Diagram 2.1 shows the normal vertebral configuration. The ilium on each side of the body is positioned beside the 27th vertebra (i.e. there are 26 bilateral pre-pelvic vertebral arches). Diagram 2.2 shows unilateral caudal shift: The left ilium is positioned beside the 28th vertebra, such that there is one extra pre-pelvic vertebral arch on that side of the body (i.e. there has been a caudal shift of the left side of the pelvic girdle). The positions at which there is a change in the alignment of the vertebral arches, and the positions of the iliac crests, are offset (i.e. at different levels on the two sides of the body).
Diagram 2.3 shows unilateral cranial shift. The ilium on the left side of the body is positioned beside the 26th vertebra, such that there is one fewer pre-pelvic vertebral arch on that side of the body (i.e. there has been a cranial shift of the left side of the pelvic girdle).
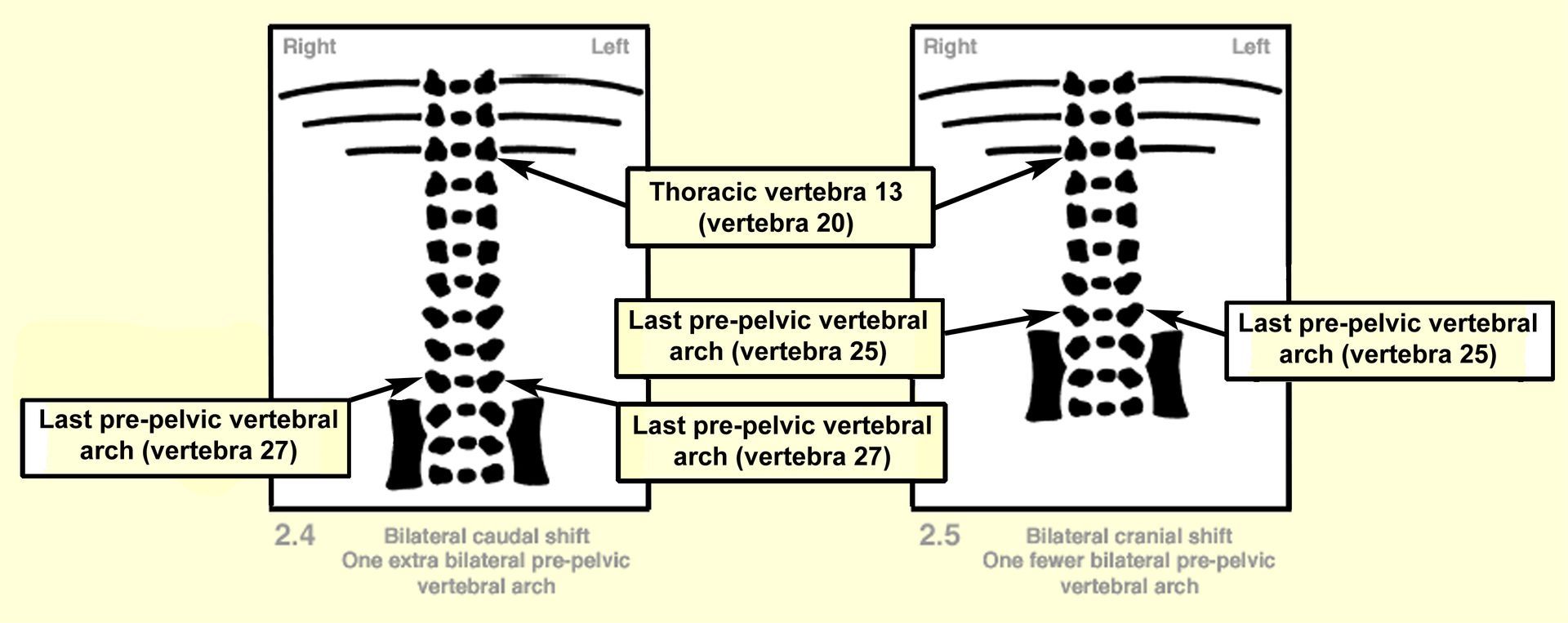
Occasionally, the iliac alignment is slightly changed without an apparent change in alignment of the associated vertebral arches (i.e. the movement is less than the length of one vertebra). Caution should be exercised if such a change is recorded, as this extent of change could be a result of movement induced during skeletal preparation.
Where unilateral or bilateral pelvic shifts occur, it is usual for pelvic articulation to occur one vertebra higher (cranial shift) or one vertebra lower (caudal shift) than expected. However, it is possible for the position of pelvic articulation to be displaced by more than one vertebra. Therefore, so that the extent and laterality of the change are known, it is recommended that the change in number of pre-pelvic arches is always stated in the description (e.g. one fewer left pre-pelvic vertebral arch, or two extra bilateral pre-pelvic vertebral arches).
For ease of data recording, the authors recommend that the sacrum is considered to comprise four sacral vertebrae; thus the sacrocaudal border will usually be between vertebrae 30 and 31.
Caudal vertebrae: It is recommended that the number of caudal vertebral should be counted. The rabbit fetus on Day 29 of pregnancy should have ossification present in at least 14 vertebrae (the exact minimum number for the strain used should be determined).
Counting of ribs is also very useful and complements counting of pre-pelvic vertebrae. Discrepancies in rib numbers are helpful clues in evaluation of vertebral changes. Also, 'supernumerary' ribs can occur at either or both of the cervicothoracic and the thoracolumbar borders.
When supernumerary ribs occur at the cervicothoracic border, they are almost always associated with the last cervical vertebra (number 7) and, as a consequence, are commonly described simply as 'cervical ribs'. In the event that ribs are associated with any cervical vertebra other than number 7, it is strongly recommended that the location of the rib (e.g. associated with cervical vertebra 'n') is included in the description. Cervical ribs are often very small and difficult to detect, because they can be obscured by other structures if the fetus is not viewed at an appropriate angle. To ensure consistent observation of these structures, it is important also that the soft tissue at the cervicothoracic border is thoroughly 'cleared' during skeletal processing.
When supernumerary ribs occur at the thoracolumbar border, the assignment of a vertebra to a particular category can be difficult. For most practical purposes the anatomy of the vertebra will not be sufficiently obvious to allow definitive categorization; consequently, the use of the term thoracolumbar rib has been recommended. As some anatomical conventions define the term thoracic as rib-carrying, it is logical to regard a vertebra with a rib as thoracic and the authors recommend that any vertebra with a rib of approximately normal length should be regarded as thoracic. If the supernumerary rib is short or rudimentary, it is generally considered that the associated vertebra can be regarded as lumbar and that the 'rib' might eventually be incorporated into the transverse process. However, there might be occasions when it is clear that a vertebra with a normal length ('long') supernumerary rib has the anatomical features appropriate to the lumbar region. If the vertebral anatomy can be adequately distinguished, it should be used to categorize that vertebra and should override any identification based on rib length, although such a decision might only be conclusive in cases where the cartilage has been fully revealed by staining.
While it is quite common to find structures which are incompletely ossified, it is less usual to find structures which show increased ossification. Fetal examiners must be aware of the natural bias towards acceptance of incompleteness as a noteworthy finding, while discounting examples where ossification is greater than would normally be expected. The latter observation should be recorded, however, where appropriate.
Observations that might indicate increased ossification are:
- Where the extent and/or density of the stain in a structure is greater than expected.
- Where structures or parts of structures which are not normally ossified show stain uptake.
The presence of a bridge of ossification between two structures which eventually fuse (e.g. adjacent sternebrae) can also represent increased ossification. It is recommended that observations relating to fusions are described as they appear and judgement as to whether they might represent advanced development is made at a later data evaluation stage.
It should be recognized that, because the assessment of the fetus describes a snapshot of time, a timeslice taken from a continuum, it follows that for a specific skeletal element the normal appearance at the gestational age being examined could represent a state of increased ossification when seen in a slightly younger animal, or conversely it could represent a state of incomplete ossification for a slightly older animal. To determine which is the correct interpretation will require consideration of post-conceptional age.
Increased ossification can have serious consequences, most likely through premature cessation of growth. Examples of structures which might be affected in this way are cranial bones, the sternum and long bones. In the cranium, premature ossification can lead to arrested growth of the skull and hence physical restriction of brain growth. In the sternum or in long bones the mostly likely result is shortening of the mature structure.
Learning objective: The Terminology in Developmental Toxicology glossary is an invaluable document. Review the skeletal section and become familiar with the terms described.
LEARNING OUTCOME EXCERCISES
1. Using your own specimens find examples of the following observations and record them using an image, simple diagram or description. As well as reinforcing the recognition levels used by your laboratory, any record provides further evidence of your examination training.
Skull bone observations e.g. incomplete ossification, unossified areas, holes, fusions, fissures.
Any of the following cervical, thoracic, lumbar and sacral vertebral arch observations:
- Reduced in size
- Misaligned
- Absent
- Incompletely ossified
- Not ossified
- Fused
Any of the following thoracic, lumbar and sacral vertebral centrum observations.
- Asymmetrically ossified
- Misaligned (ossification)
- Absent
- Not ossified
- Unilateral ossification
- Bipartite/unfused ossification centres
- Dumbbell shaped/incompletely fused ossification centres
- Incompletely ossified
- Fused
Any observations on caudal vertebrae
In the normal rabbit skeleton, 12 pairs of ribs are present, associated with the thoracic vertebrae.
What are your criteria for recording rib observations?
Are these observations used?
- Thickened rib
- Fused/bifurcated rib
- Cervical/ thoracolumbar supernumerary ribs (how are these defined? Length? appearance?)
Any observations relating to the pectoral and pelvic girdles.
Any of the following sternebral observations:
- Incompletely ossified
- Bipartite
- Misaligned
- Misshapen
- Not ossified
- Fused
- Additional ossification centre(s)
Any observations relating to the fore and hindlimbs.
2. Rollover images of normal specimens to test knowledge of bones
Each of the hyperlinks below will display an area of the skeleton. Blank labels point to some of the important structures. The pages have been sized so that you can print them, fill out the labels and then check your answers by pointing your mouse arrow at each label.
3. Quick crossword!
The fetal rabbit skeleton is relatively well ossified at term. However, certain bones often show incomplete or non ossification and higher incidences of these observations may indicate a delay in skeletal development. Look at the crossword and work out the identity of the bones from the clues - are higher incidences of incomplete or non ossification of these bones considered to be indicators by your laboratory?
REFERENCES
Aliverti V, Bonanomi L, Giavini E, Leone VG, Mariani L
The extent of fetal ossification as an index delayed development in teratogenic studies in the rat.
Teratology 20,237-242 1979
Ariyuki F
A study of the fetal growth retardation in teratological tests:- relationship between bodyweight and ossification of coccygeal vertebrae in mouse and rat foetuses.
Teratology 22, 43-49 1980
Ariyuki F, Ishihara H, Higaki K, Yasuda M
A study of foetal growth retardation in teratological tests: Relationship between growth and ossification of the skeleton in rat foetuses.
Teratology 26, 263-267 1982
Ariyuki FH, Higaki K, Yasuda M
Staging of ossification in the supraoccipital bone in the preterm rat foetuses.
Congenital Anomalies 20, 275-381 1980
Beck SL
Prenatal ossification as an indicator of exposure to toxic agents.
Teratology 40, 365-374 1989
Beyer PE, Chernoff N
The induction of supernumerary ribs in rodents: role of maternal stress.
Teratogenesis,Carcinogenesis and Mutagenesis 6, 419-429 1986
Black DL
Role of maternal toxicity in assessing developmental toxicity in animals: a discussion.
Regulatory Toxicology and Pharmacology 16,184-201 1992
Boardman JP, Mitala JJ
Cartilage staining technique for the examination of unskinned foetal rat specimens previously processed in Alizarin Red S.
Teratology 30,383-384 1984
Chernoff N Kavlock RJ Beyer PE Miller D
The potential relationship of maternal toxicity, general stress and fetal outcome.
Teratogenesis, Carcinogenesis and Mutagenesis 7, 241-253 1987
Chernoff N, Rogers JM, Turner Cl, Francis BM
Significance of supernumerary ribs in rodent developmental toxicity studies: postnatal persistance in rats and mice.
Fundamental and Applied Toxicology 17,448-453 1991
Chernoff N, Woodrow Setzer R, Miller DB, Rosen MB, Rogers JM
Effects of chemically induced maternal toxicity on prenatal development in the rat.
Teratology 42,651-658 1990
Dawson AB
A note on the staining of the cleared specimens with Alizarin Red S.
Stain Technology 1,123-124 1926
Ehrenhaft JL
Development of the vertebral column as related to certain congenital and pathological changes.
Surgery Gynecology and Obstetrics 76, 282-292 1943
Fritz H, Hess R
Ossifications in the rat and mouse in the perinatal period.
Teratology 3, 331-338 1970
Fritz H
Prenatal ossification in rabbits as indicative of foetal maturity.
Teratology 11,313-320 1975
Gray H
Gray's Anatomy.
Longmans Greene and Co 1913
Greene EC
Anatomy of the rat.
Hafner, New York 1959
Hall BK
The embryonic development of bone.
American Scientist 76,174-180 1988
International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Detection of Toxicity to Reproduction for Medicinal Products, June 1993
Igarashi E, Kawamura N, Okumura H, Hotta T, Okamoto M, Inaoka S, Yasuda M
Frequency of spontaneous axial skeletal variations detected by the double staining technique for ossified and cartilaginous skeleton in rat fetuses.
Congenital Anomalies 32, 381-391 1992
Inouye M
Differential staining of cartilage and bone in fetal mouse skeleton by alcian blue and alizarin red S.
Congenital Anomalies 16, 171-173 1976
Kavlock RJ, Chernoff N, Rogers EH
The effect of acute maternal toxicity on fetal development in the mouse.
Teratogenesis, Carcinogenesis and Mutagenesis 5, 3-13 1985
Kawamura S
Bone staining for fetal rat and rabbit specimens without skinning and removing adipose tissue.
Teratology 40, 680 1989
Khera KS
Maternal toxicity: a possible etiological factor in embryofetal deaths and fetal malformations of rodent-rabbit species.
Teratology 31, 129-153 1985
Kimmel CA, Trammel C
Rapid procedure for routine double staining of cartilage and bone in foetal and adult animals).
Stain Technology 56,271-273 1981
Kimmel CA, Wilson JG
Skeletal deviations in rats: malformations or variations?
Teratology 8, 309-316 1973
Kimmel CA, Laborde JB, Trammel CT
Evaluation of cartilage and bone formation in fetal skeletons following prenatal insult reveals abnormalities not apparent in alizarin stained specimens.
Teratology 25, 54A-55A 1982
Koskinen L
A note on craniofacial sutural growth.
American Journal of Physical Anthropology 45 (3), 511-516 1976
Massler M, Schour I
The growth pattern of the cranial vault in the Albino rat by Alizarin Red S staining.
Anatomical Record 105, 83-101 1951
Nijweide PD, Burger EH, Feyen JMH
Cells of bone: proliferation, differentiation and hormonal regulation.
Physiological Reviews 66, 4, 855-886 1986
Organization for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals, Test Guideline No. 414, Prenatal Development Toxicity Study, January 2001
Yasuda M, Yuki T
Colour atlas of fetal skeleton of the mouse, rat and rabbit.
Ace Art Co Ltd 1996
Yamada T
Selective staining methods for cartilage of rat fetal specimens previously treated with Alizarin Red S.
Teratology 43,615-619 1991
Wilson JG, Warkany J, eds.
Teratology: Principles and Techniques.
Chicago: University of Chicago Press 185-213 1965
Wise LD et al
Terminology of Developmental Abnormalities in Common Laboratory Animals (Version 1).
Teratology 55, 249-292 1997
Whitaker J, Dix KM
Double staining technique for rat foetus skeletons in teratological studies.
Laboratory Animals 13,309-310 1979
United States Environmental Protection Agency
Health Effects Test Guidelines
OPPTS 870.3700 Prenatal Developmental Toxicity Study, August 1998
Trueman D, Jackson SW and Trueman, B
An automated technique for double staining rat and rabbit fetal skeletal specimens to differentiate bone and cartilage.
Biotechnic and Histochemistry 74 (2), 98-104 1998
Thorogood
The developmental specification of the vertebrate skull.
Development 103, 141-1531988
Strong RM
The order, time and rate of ossification of the albino rat skeleton.
American Journal of Anatomy 36,313-351 1925
Popesko P, Ratova V, Horak J
A colour atlas of the anatomy of small laboratory animals
Volume 2, The rat, mouse, golden hamster. Wolfe 1992
Rowett HGQ
Dissection guides III, The rat.
John Murray 1951
Rowett HGQ
The rat as a small mammal
Third edition. John Murray 1974
Sarnat BG
Something of the nature of gross sutural growth.
Ann Plast Surg 17 (4), 339-349 1986
Simpson E
Growth factors which affect bone
TIBs 427-530 1984
