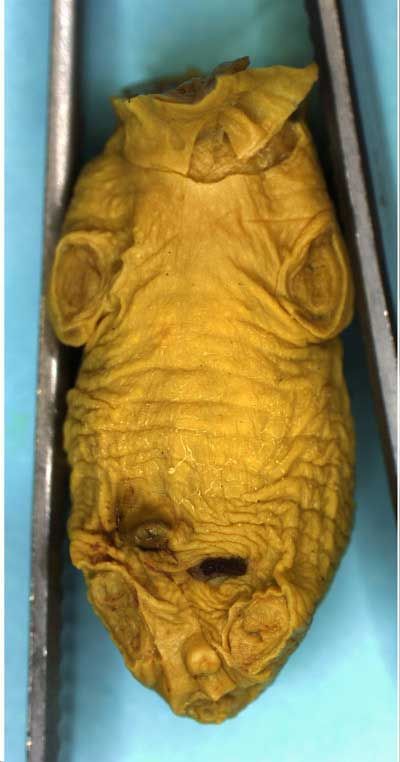
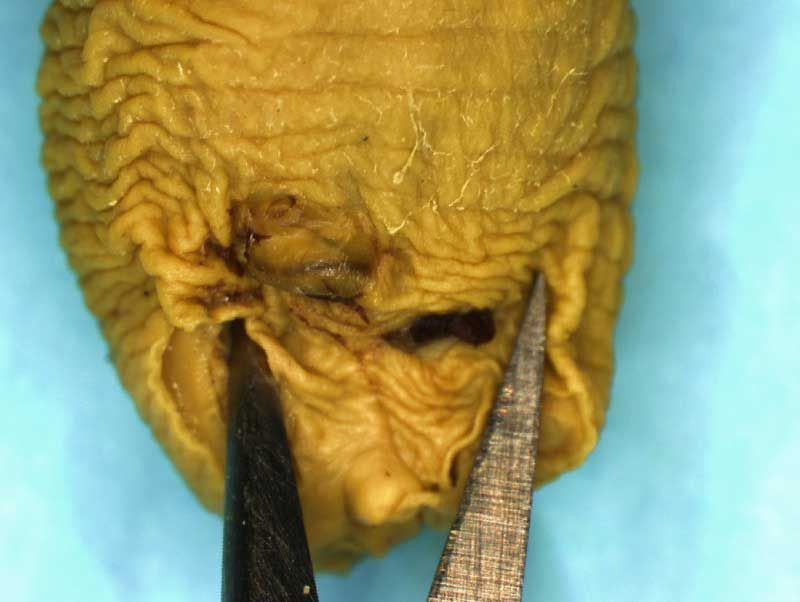
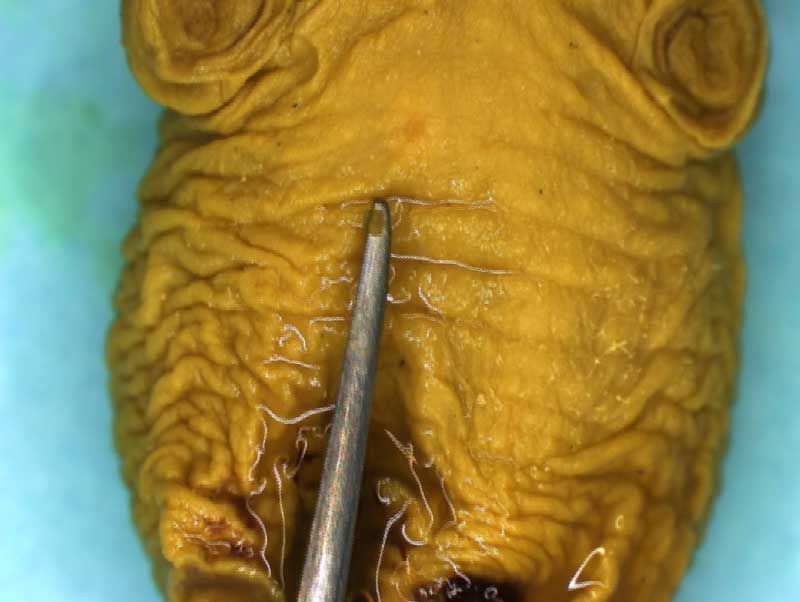
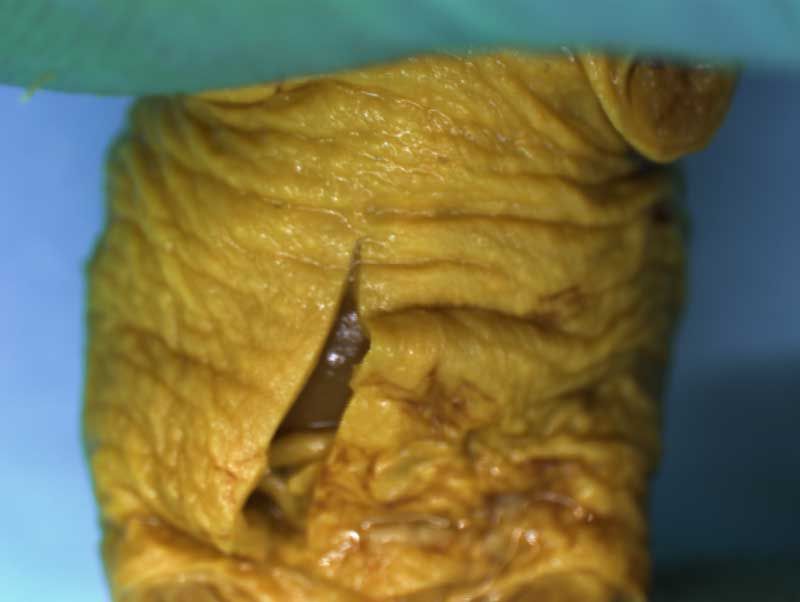
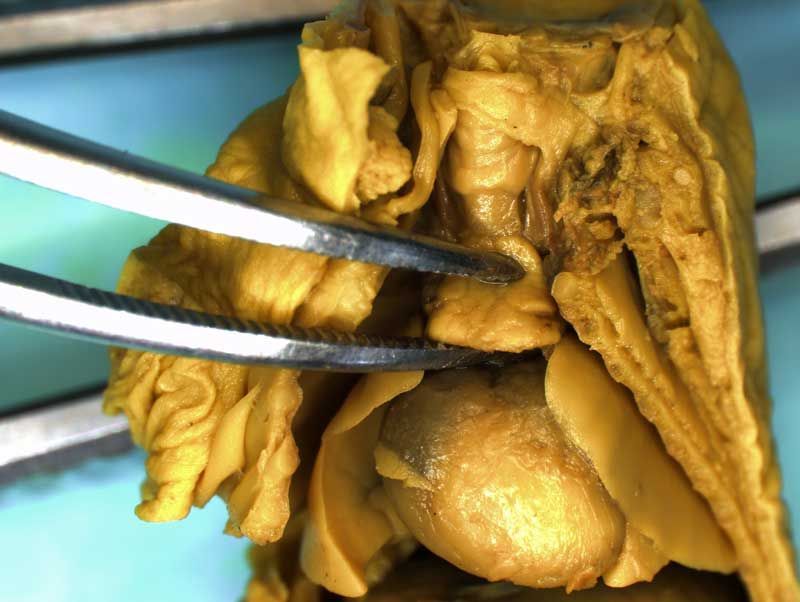
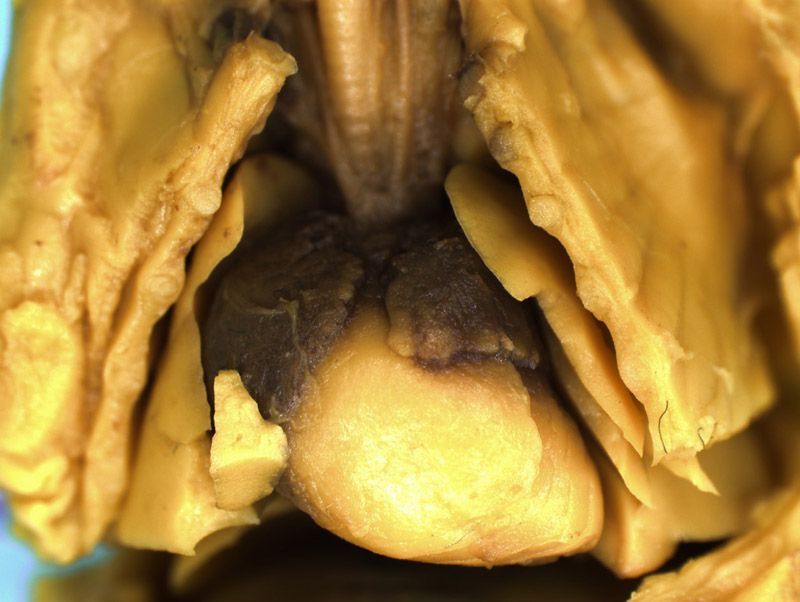
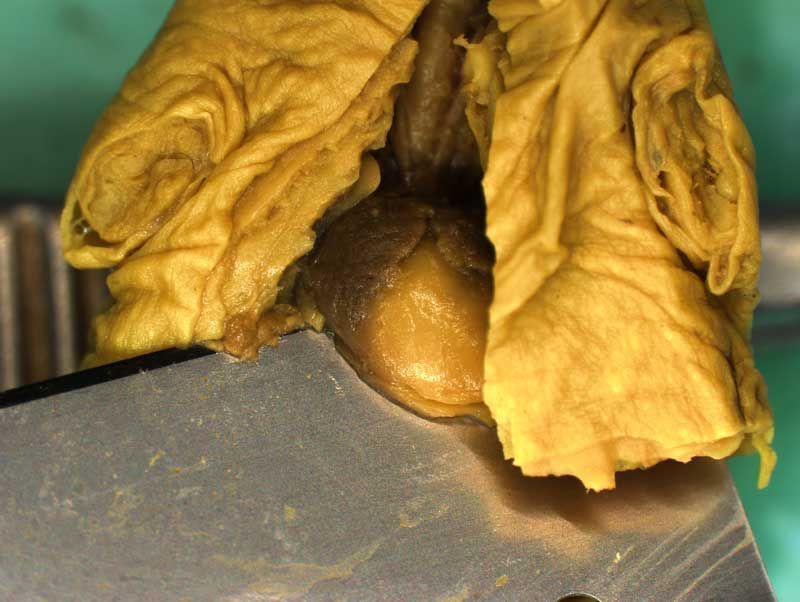
Rat Fixed Head (WILSON SECTION) and body (MICRODISSECTION)
RAT FIXED HEAD (WILSON SECTION) AND BODY (MICRODISSECTION) EXAMINATION - INTRODUCTION
Why are fetuses examined?
The aim of regulatory reproductive toxicity studies is to reveal any effect of an active substance on mammalian reproduction. In one of the studies designed to achieve this aim pregnant animals are treated during the period of embryonic/fetal development.
Current International Conference on Harmonisation (ICH), Organisation for Economic Co-operation and Development (OECD) and the US Environmental Protection Agency (EPA) guidelines require that these embryofetal development studies are performed in 2 laboratory animal species (one rodent and one non-rodent), and that fetuses from the treated dams are examined for developmental and structural abnormalities, by soft tissue and skeletal examination techniques.
The rat is commonly chosen as the rodent species and the current guidelines, referred to above, specify that "a minimum of 50% rat fetuses should be examined for visceral alterations, regardless of the technique used."
Why is technique used?
There is a regulatory requirement to examine the external surface of the fetus together with the internal structures of the head (for example detail of the eyes, brain, nasal passages and tongue) and the thoracic and visceral organs, in rat specimens.
Advantages of the Bouin's fluid fixed tissue serial sectioning (Wilson) and microdissection techniques:
- Facilitates examination of internal structures, as required by regulatory guidelines, for structural abnormalities and developmental delay.
- As the technique uses fixed tissues, examination of the specimens is at the convenience of the examiner.
- The technique allows:
- retention of tissue for future reference/further examination.
- further investigations are possible, for histopathological confirmation of lesion.
- It penetrates the tissue well, without causing overt hardening.
- Fixes rapidly.
- Provides a permanent yellow colour to the fixed tissue so providing clarity in distinction between structures.
- Is sufficiently acid that it acts as a decalcifying agent, which facilitates sectioning through skull bone.
- The 3D appearance of the specimen makes understanding of the spatial relationship of different structures with each other relatively easy.
Training to examine Bouin's fluid fixed specimen using Wilson section and microdissection techniques
Ideally the trainee should be provided with a training plan and the date of any training received should be recorded. Regular review of the trainee's progress is essential.
SOPs and user guides should be made available as training materials. These should be referred to, along with any other relevant materials, such as this training web, during training. Adherence to Good Laboratory Practices should be evident at all times.
Initial teaching sessions are ideally organised on a one-to-one basis, interspersed with time allowed for the trainee to practice, consolidating what has been learned. Wherever possible, the use of materials from studies that have already been evaluated will avoid time pressures at this stage.
Once this initial phase has been completed, training can then be continued 'on-study' with close supervision by experienced examiners. Initially, training should include 100% review of the examinations performed by the trainee, gradually reducing as competency is acquired. The time taken to achieve competency will naturally depend on work throughput, as much as on the aptitude of the trainee, and it might be necessary to revert to more exhaustive checking if checking/review reveals an increasing level of inaccuracy or inconsistency. It is expected that the trainee would have 18 months experience in the technique, and have evaluated a minimum of 500 litters before they are be considered fully competent.
It is recommended that all structures listed on the The International Register of Fetal Morphologists (IRFM) Expected Minimum Structure List for Fetal Morphology Examinations document be examined and that the Fixed head and body examination (Wilson section) - key structures and notes document be used as a reference.
Learning objectives:
- Familiarise yourself with your training objectives
- Review 'in house' training material
- Review UK IRDG recommended minimum requirements for fetal examinations document
REQUIREMENTS FOR EXAMINATION
Any procedure using Bouin's fluid should be performed in a well ventilated area. For the examination of head sections, low-power magnification is recommended. A good light source is also essential with the specimen being illuminated from above. The setup pictured below demonstrates illumination from above using the flexible necks of a cold light source.
The specimen can be moved and manipulated using forceps, however, the examiner should find what is comfortable for them whilst allowing free movement.
Since there is no absolute and wholly objective standard against which to evaluate specimens, every effort should be made to assess the anatomy in exactly the same way for every fetus, applying identical criteria to distinguish 'normal' from 'abnormal'. It is recommended that measures to ensure a consistent approach be built into any laboratory's work practice. Working methodically should increase the consistency of examination and limit the likelihood of omissions. Whether all examiners in a laboratory should be required to follow the same routine or be allowed to develop their own is a matter for individual preference, but some degree of consistency is desirable.
Remember:
Magnification
- Binocular microscope, x6 minimum
Light source
- Preferred - swan neck cold light source
Dissection equipment
- Forceps (curved or straight)
- Pointed fine forceps (curved or straight)
- Thin (razor type) blades
Examination dishes
- Suitable clear shallow container, able to hold all head sections
Laboratory work station
- In quiet, well ventilated room, specific for purpose
- Adequate space for:
- manipulation of specimen under examination
- whole litter being examined
- container for medium in which specimens are examined e.g. industrial methylated spirits
- labelled transfer containers, if appropriate, filled with appropriate fixative (if required)
- paper towels
- data collection system, paper records or monitor and keyboard
- Suitable chair
- Comfortable, safe
- 3 way adjustable
- Good overhead lighting
Protective clothing
- Safety glasses
- Protective gloves
- Laboratory coat
Access to reference material:
- The International Register of Fetal Morphologists (IRFM) Expected Minimum Structure List for Fetal Morphology Examinations
- Terminology in Developmental Toxicology glossary
- Company training guides, including recognition levels employed
- Company SOPS
- Examples of normal specimens

PREPARATION OF SPECIMENS
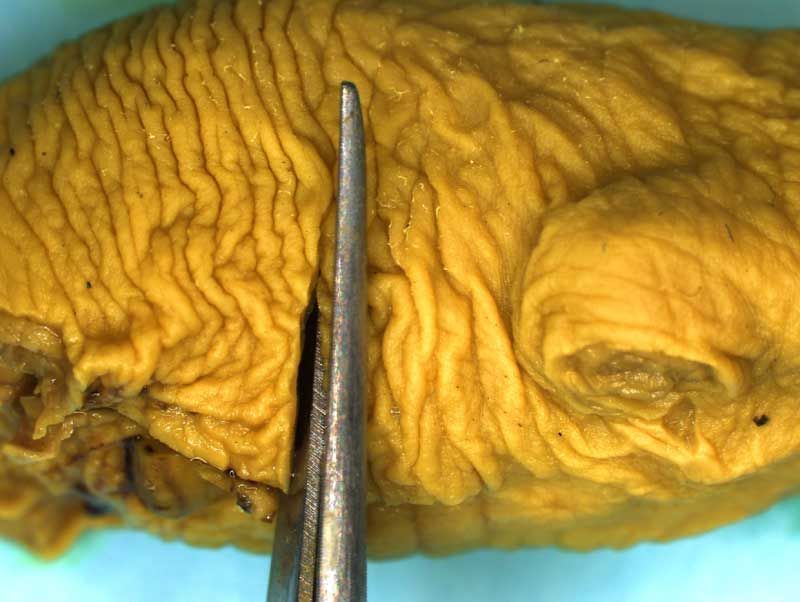
Unskinned rat fetuses are fixed whole in Bouin's fluid and examined externally before being decapitated prior to head sectioning and microdissection. The limbs and tail may also be removed, if desired, as illustrated below to facilitate grip and manipulation by the examiner.

Bouin's fluid is a mixture of saturated aqueous picric acid, formaldehyde and glacial acetic acid. Care must be taken when preparing Bouin's fluid as the picric acid solution must not be allowed to dry out - it is explosive when dry!
The precise mechanism of action of this fixative is unknown, it penetrates the tissue well, fixes rapidly, and provides a permanent yellow colour to the fixed tissue so providing clarity in distinction between structures. It is sufficiently acid to act as a decalcifying agent, which facilitates sectioning through skull bones. The fixation process takes place quite satisfactorily at room temperature in 7 - 14 days. Rat bodies may require hardening before sectioning and this can be accomplished by placing in alcohol for 24 hours before sectioning. Specimens taken straight from fixative for sectioning should be washed in either alcohol or water to remove excess Bouin's fluid.
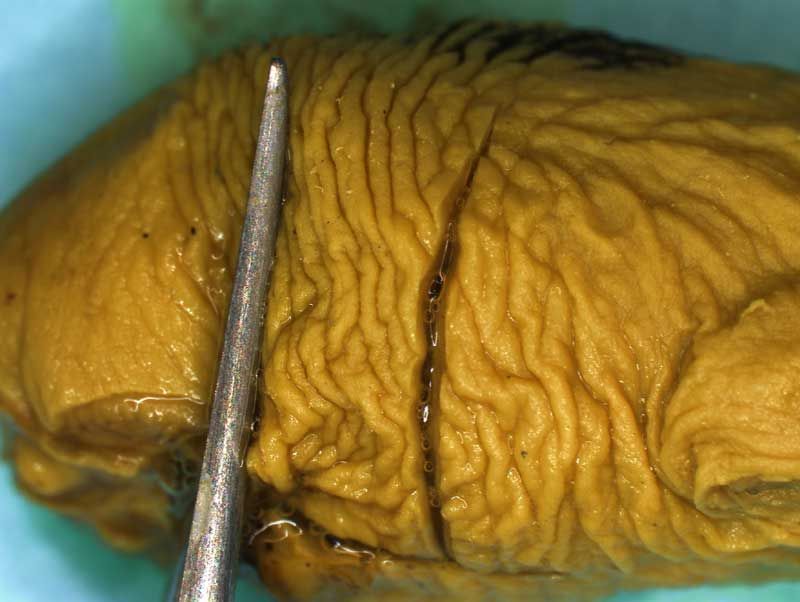
Depending on laboratory guidelines approximately 7 -12 coronal sections are prepared by freehand serial sectioning of the head using a thin blade. The lower jaw can be left in situ or removed before sectioning.
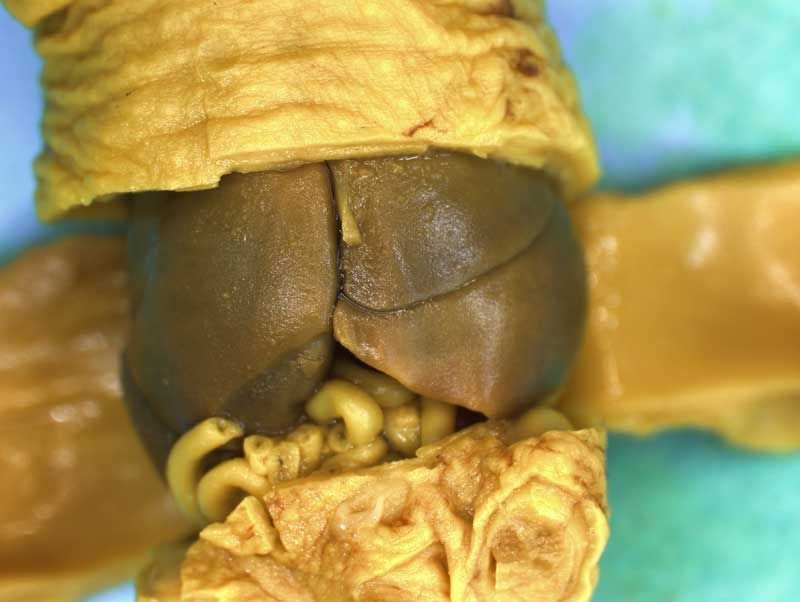
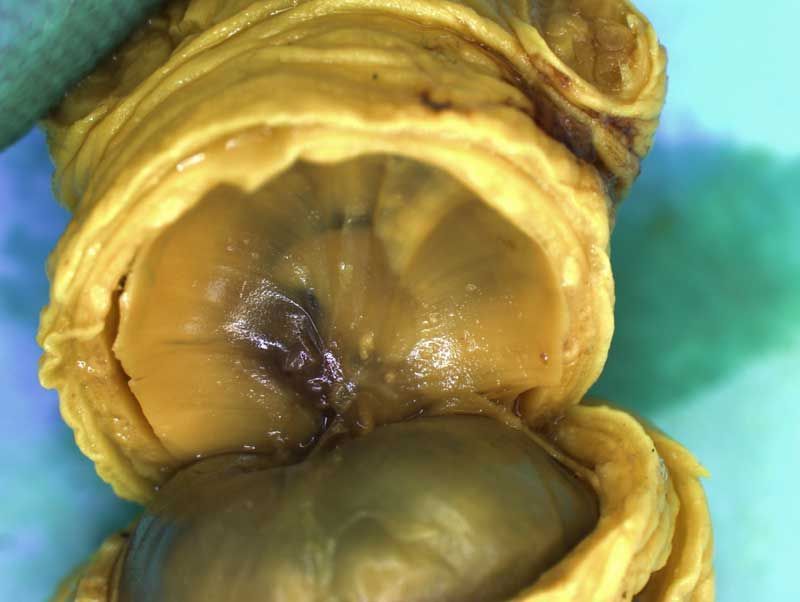
Incisions should be made to expose the contents of the abdomen. Following examination of the diaphragm another incision is made to facilitate examination of the contents of the neck and thoracic cavity. Careful dissection (blunt and sharp) should be used to expose and move tissues during the examination process. Blood vessels should be traced and their patency assessed. The internal structure of the heart and kidneys should be examined. The intestine should be checked for areas of constriction and blockage. The sex of the fetus should be assessed by examination of the gonads and should confirm that, which was determined externally.
Main incisions/sections made during dissection process:
Learning objective: review the fixation method used in your laboratory. Review the methods outlined by Wilson (1965) and Barrow and Taylor (1969) - compare and contrast these with the sectioning and dissection methods used in your laboratory.
ASSESSMENT OF NORMAL
It is considered important to remember that if the examination data are collected inaccurately, inconsistently, or with insufficient detail, meaningful interpretation of these data is impossible. The examination of fetal specimens is therefore a crucial step in the evaluation of reproductive toxicity studies. The importance of this stage in data evaluation cannot be overstated.
Recognition of the 'normal' appearance
Laboratory recognition levels are the cut-off points between what is considered to be 'normal' and what must be recorded as 'abnormal' within a particular laboratory over a particular time period. Also, where observations are qualified by estimates of extent ('severity'), recognition levels provide the cut-off points between severities (advice on the recording of severity is given below). Translated into practical terms, they are criteria enabling the examiner to make consistent decisions at the borderlines between different categories.
Between laboratories there can be differences in the recognition levels set for some observations. It would be difficult to attempt 'standardisation' across laboratories in this area, because this would require setting recognition levels at an unacceptably coarse level in order to allow for the many factors that lead to inconsistency (including strain of animal, time of necropsy, laboratory environment and procedures). The priority should be ensuring consistency within each laboratory, so that valid comparisons can be made between treated and control animals, both within and between studies conducted in that laboratory.
Deciding where to draw the line between what is considered unremarkable and what must be recorded as an observation is one of the most difficult problems in fetal examination. In all biological systems there is natural variation and very few individuals in a population, or in a population sample, will have a body conformation that complies precisely with any idealised textbook description (the anatomical norm or anatomical standard). Most individuals will have an appearance that differs slightly from the anatomical norm, but which does not do so sufficiently, nor so infrequently, as to be regarded as 'abnormal'. This range of conformity can be considered to constitute the population norm.
Each laboratory uses recognition levels in the recording of fetal abnormalities, which define the boundaries between what is considered to be normal and what is abnormal within that laboratory, and also in many cases the boundaries of the qualifications used to describe the extent of an abnormality.
Other considerations - Assessing mechanical damage and processing artefacts
It is possible for mechanical damage to occur at necropsy e.g. subcutaneous haemorhage.
It is strongly recommended that any damage known to have occurred during the stages before fetal examination is recorded and that appropriate notes are made available to the examiner. Where conditions observed at evaluation are suspected but not confirmed to be artefactual, evaluation requires a common-sense approach. Examples of questions to be considered are:
- Are the margins of the structure smooth or jagged, as if shearing has taken place?
- Can the pieces be 'jig-sawed' back together?
- Has the fixation caused any shrinkage?
- Is the structure an unusual colour?
- Has the specimen not been completely immersed in fixative, or have the specimens clumped together, causing non-uniform penetration of the fixative?
Other considerations - section quality
It is possible when the specimens are examined that all of the key structures are not clearly visible. If this is the case it is important to assess whether this is due to a true structural abnormality or to the quality of the section. To clarify this situation rebuilding of the head sections and re-sectioning of the appropriate area should be performed.
Where clarification is required examples of questions to be considered are:
- Is there apparent asymmetry of bilateral structures?
- Do small structures appear to be absent?
- Do structures appear to be displaced?
- Do structures appear to be misshapen?
Learning objective: review the Van Julsingha (1977) paper to read more about artefacts e.g. cystic dilatation in the brain.
ASSESSMENT OF DIFFERENCES FROM NORMAL
Changes from normal should be recorded according to your own laboratory's standards, with references to internal recognition levels, using the appropriate SOPs, training guides, user manual and any other relevant training information.
'Severity' of observations
It is recommended that some means of categorising the degree of change or extent of an observation be devised to aid in interpretation. Commonly used systems use qualifiers such as minimal (or slight), moderate and marked (or severe). Defining these terms without specific examples is difficult, but the following general principles might be suitable:
- Minimal: Apparent at close scrutiny
- Moderate: Apparent without close scrutiny
- Marked: Strikingly obvious
Not all observations need to be qualified by severity, but where a single term might be descriptive of a wide range of severity (from a condition which is only slightly outside the normal range to one which is potentially much more harmful), a qualifier indicating severity is strongly recommended. Examples of such terms are: misshapen, displaced, enlarged, reduced in size, shortened and widened.
Where severities are used, they should be noted at the examination stage, but where appropriate they can be merged or rationalised at a later data evaluation stage. The data must be assessed carefully to ensure that such a rationalisation will not obscure or exaggerate any findings.
Learning objective: The Terminology in Developmental Toxicology glossary is an invaluable document. Review the visceral section and become familiar with the terms described.
Demonstration of spatial awareness/anatomical knowledge
Demonstrate your understanding of this "3D" procedure by thoroughly mixing up the head sections and then arranging them in the correct order/orientation.
Key learning point exercises and questions
Give 3 reasons why Bouin's fluid is effective as a fixative for visceral examination.
Name 3 requirements that should be in place in the lab before examination of Bouin's fluid fixed tissues can take place.
What protective clothing should you wear when performing examination of Bouin's fluid fixed tissues?
Where would you find a list of the head and body areas that should be looked at during external/visceral examination
Use your own specimens to find examples, if possible of:
- Cleft palate
- Retinal fold
- Subdural haemorrhages
- Absent/small thyroid gland
- Any observations relating to the thymus gland
- Any observations relating to the aortic arch/pulmonary trunk/ductus arteriosus.
- Right subclavian artery arising from aortic arch (absent innominate artery)
- Ventricular septal defects
- Do you record severity levels?
- Do you make the distinction between the membranous and muscular septum?
- Any observations relating to the lungs
- Diaphragmatic hernia
- Anomalous confluence of caudal vena cava with left hepatic vein
- Any observations relating to the liver
- Any observations relating to the kidney and ureter
- Do you have severity levels for kidney and ureter observations?
- Any observations relating to the gonads
List 2 observations that may be associated with fetuses with subcutaneous oedema
List 3 observations, which would help you distinguish the abnormality situs inversus
What are your criteria for recording hydrocephaly vs. dilated lateral ventricles
REFERENCES
Barrow MV Taylor WY
A rapid method for detecting malformations in rat fetuses
J Morph 127 291-305 1969
Beck F
Evaluation of organs (gross organ pathology)
Methods in prenatal toxicology ed Neubert D Georg Thieme Stuttgart 1977
Flaherty JF
Stain Technol 47 2 53-58 1972
Surface staining of 1MM Wilson slices of foetuses for internal visceral examination
Ikemi N Otani Y Ikegami T Yasuda M
Palatal ruga anomaly induced by all-trans-retinoic acid in the Crj:SD rat: possible warning sign of teratogenicity
Reproductive Toxicology 15 87-93 2001
Olds RJ, Olds JR
A colour atlas of the rat dissection guide
Wolfe medical publications Ltd
1979
Peters PWJ
Surface staining of Wilson razor blade slices
Methods in prenatal toxicology ed Neubert D Georg Thieme Stuttgart 1977
Rowett HGQ
Dissection guides IV the rabbit
John Murray 1952
Rowett HGQ
Dissection guides III the rat with notes on the Mouse
John Murray 1951
Stertz H, Lehmann H
A critical comparison of the freehand razorblade dissection method according to Wilson with an in situ sectioning method for rat fetuses
Tera Carc Mutag 5 347-354 1985
Van Julsingha EB, Bennett CB
A dissecting procedure for the detection of anomalies in the rabbit fetal head
Methods in prenatal toxicology ed Neubert D Georg Thieme Stuttgart 1977
Whitehouse RH Grove AJ
The dissection of the rabbit with an appendix on the rat
University tutorial press Ltd 1933
Wilson JG
Methods for administering agents and detecting malformations in experimental animals
Teratology Principles and Techniques 262 277 1965
Yasuda M Ohya R Sato TJ Inoue N
Variations in the palate rugae in the mouse as an indicator for detection of teratogenicity
J Toxicol Sci 17 348 1992